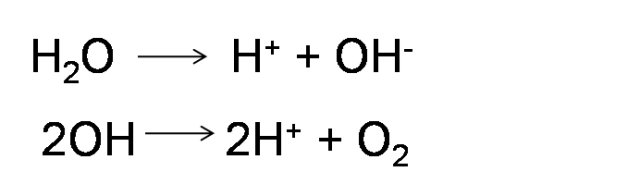
Practical-2
AIM :-
Study of Plasmolysis through the effect of Isotonic, Hypotonic as well as Hypertonic Solutions
of Sodium Chloride and Potassium Chloride on Mounted Epidermal Cells of Tradescantia leaf.
INSTRUMENTS AND MATERIALS : -
Slide, Cover slip, Microscope, Dropper, Blotting paper, Tradescantia leaf, Water, Glycerine, NaCL solution, KCL solution.
| Plasmolysis through the effect of Isotonic for std 10 to 12 |
Conclusion :-
This a kind of exosmosis. When living plant cell is placed in a concentrated solution of sugar or salt plasmolysis is induced in them. Normally living cells are turgid as concentrated of vacuolar sap is lower than the surrounding solution water from the cell starts moving outside through exosmosis.